
A novel ‘‘anchoring flap’’ (FCSEMS-AF) system made of four flaps in the proximal end to prevent anti-migration. One proximal lasso with gold marker is a cutting-edge advance in therapeutic endoscopy for benign biliary diseases as inversion retrieval.
The device is indicated for palliative treatment of bile duct malignant and/or benign strictures.
| Features | Benefits |
|---|---|
| Flaps in proximal ends | Anchorage to the choledochal wall and preventing both proximal and distal stent migration* |
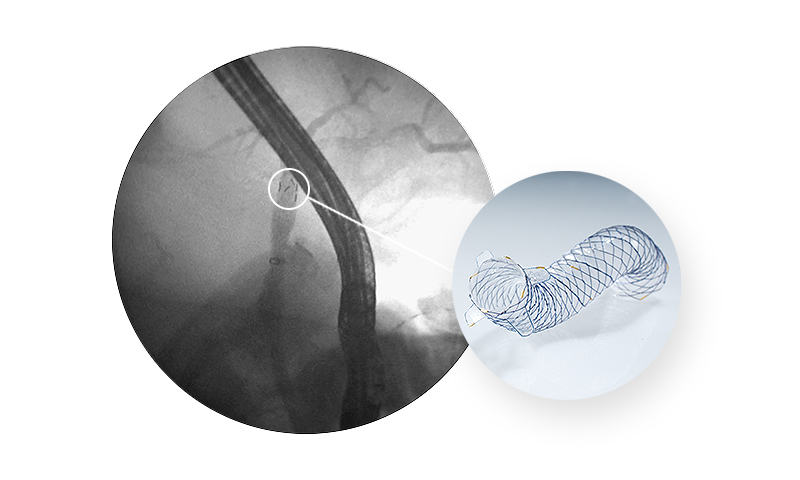 |
|
| Lasso with Gold | Stent inside-out removal |
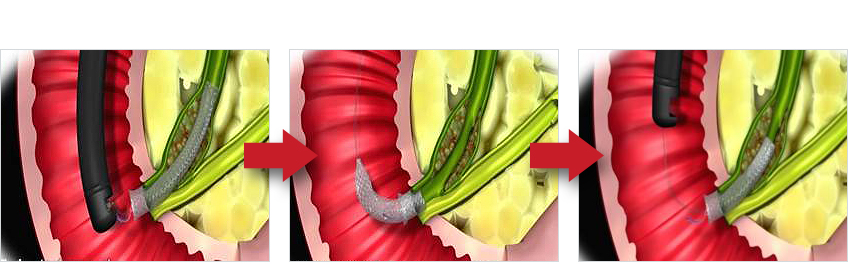
※ Alternatively using a snare to remove |
|
| Clinical Study | B. Mangiavillano., et al1 | Do Hyun Park, et al2 |
|---|---|---|
| Migration Rate | 3.3% (n=1 of 32) One at 125 days after placement | 0% (n=22) |
| Successful Removal Rate | 93.7% (n=30 of 32) One of the two patients in whom stent removal was not attempted died on day 122 after stent placement from underlying cardiovascular disease. The leak had clinically closed, and the abdominal drain had been removed. One patient was lost to follow-up | 100% (n=22%) |
| Stent(mm) | Delivery Device | ||||
|---|---|---|---|---|---|
| Diameter | Usable Length* | Total Length | Length(mm) | Diameter(mm/fr) | |
| BCT-08-040-180 | 8 | 23 | 40 | 1800 | 3.4/10.2 |
| BCT-08-120-180 | 103 | 120 | 1800 | 3.4/10.2 | |
| BCT-10-040-180 | 10 | 23 | 40 | 1800 | 3.4/10.2 |
| BCT-10-120-180 | 103 | 120 | 1800 | 3.4/10.2 | |
※Sizing and availability varies by country
All medical devices have associated risks. Please refer to the package insert and other labeling for a complete list of indications, contraindications, precautions and warnings. For further information on the Products, please contact your local M.I.Tech(Hanarostent) Representative.